Japan Science and Technology Corporation • CREST
R&D of System Technologies for Resource Recycling and Minimum Energy Requirement
Development of high performance natural gas reforming system for residential-use fuel cells
Hydrogen Production from Natural Gas
The hydrogen energy society is expected to realize in order to overcome global warming and various energy problems. However, the quantity of hydrogen that can be produced by using renewable energies such as solar, wind, and hydraulic power is not sufficient for demands. In such situation, the utilization of natural gas and/or the production of hydrogen from the natural gas seem to be alternatives and the most realistic solution at least in the first half of twenty-first century.
For example, the development of small co-generation system using the micro-gas turbine is widely studied for the utilization of the natural gas. In addition, fuel cells are expected to be a more high-efficient power generating system. The fuel cells are anticipated to be used in residences in addition to the installation to electric vehicles. Home-use fuel cells can provide hot-water and electricity, simultaneously. To commercialize the residential-use fuel cells, it is important to establish the hydrogen production technology that supplies abundant pure hydrogen at low cost, in addition to the development of fuel cells itself. As shown in the following table, there are a couple of hydrogen production technologies from the natural gas (main constituent is methane, CH4), including steam and carbon dioxide reforming methods.
|
Steam reforming
|
CH4 + H2O → CO + 3H2
|
ΔH298 = 206 kJ/mol
|
|
CO2 reforming
|
CH4 + CO2 → 2CO + 2H2
|
ΔH298 = 247 kJ/mol
|
|
Partial oxidation
|
CH4 + 1/2O2 → CO + 2H2
|
ΔH298 = -36 kJ/mol
|
In this project, we are focusing on the partial oxidation (POX) method. This technique is exothermic reaction; therefore, fast start-up is expected compared to the steam reforming with large endothermic reaction. However, as drawbacks of the POX method, it had been accepted that resultant hydrogen concentration was fairly low in the case of usage of air as oxidant. And there is also a safety problem because methane and air should be fed into the reformer at same time. To overcome the drawbacks, we are looking at a new-type reformer that consists of oxygen permeable ceramics and a proton conductor. The schematic diagram of this reformer is shown here.
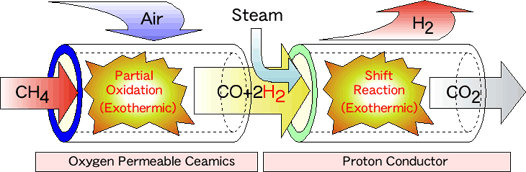
Natural gas introduced from the left is firstly reformed into syngas (H2 + CO) at the component of oxygen permeable ceramics. At which, the partial oxidation reaction occurs by using pure oxygen separated from the air by using the dense oxygen permeable ceramics. After shift conversion to increase the hydrogen content, hydrogen is then pumped out of the mixed gas by the proton conductive ceramics. The hydrogen gas stream produced here is pure and does not contain carbon monoxide which degrade the performance of proton-exchange-membrane-type fuel cells. In this reformer system, we are focusing on the development of high-performance oxygen permeable ceramics.
Oxygen Permeable Ceramics
The oxygen permeable ceramics is a mixed ionic and electronic conductor which can conduct oxygen ion and electron, simultaneously, as shown in the following figure. For this material, there is no necessity for applying voltage for oxygen transfer, since the oxygen potential gradient can be the driving force of the transportation of oxygen. That is to say, the oxygen permeable ceramics is considered to be an intelligent material by which oxygen required for the methane conversion can be automatically supplied as pure oxygen by using the oxygen potential gradient.

Obviously, the important characteristic of this membrane is an oxygen flux density that represents the quantity of oxygen permeated. The oxygen flux density is usually evaluated as an unit of mol•cm-2 •s-1, which is the mole number of oxygen permeated at unit second and per unit area. In addition, the another expression of sccm•cm-2, which is the volume of oxygen at standard condition (0 °C, 1 bar) permeated at unit minute and per unit area. Conversion between them is expressed as follows. The values are also possible to show as a current density ( mA•cm-2).
1 μmol•cm-2•s-1 = 1.34 sccm•cm-2 = 386 mA•cm-2
1 μmol•cm-2•s-1 corresponds to 1.34 sccm•cm-2. For example, perovskite-type oxides such as in La-Sr-Fe-Co, Sr-Fe-Co and La-Sr-Ga-Fe systems are well-known to be good oxygen permeable ceramics. They can exhibit an oxygen flux density of 1 ~ 8 μmol•cm-2•s-1. The oxygen flux density, which originates from mixed oxygen-ion and electronic conduction, can be theoretically calculated from the following Wagner's equation:

where σi and σel denote oxygen ion and electronic conductivities, respectively. And, T is absolute temperature, R and F are gas constant and Faraday constant, respectively. The membrane thickness of d is also important factor in the equation. The next figure shows the ionic and electronic conductivity as a function of oxygen partial pressure.

The resultant oxygen flux density of jO2 is also plotted in the case of T = 1273 K and d = 0.1 cm. In which, p- and n-type conductivities are plotted as dashed lines with a positive and negative slope, respectively, while the ionic conductivity is plateau because it is usually independent on oxygen partial pressure. Total conductivity of σtotal (shown as solid red line) is the sum of ionic and electronic conductivities. On the other hand, the ambipolar conductivity of σamb which is in the integral part of the equation and controls the oxygen flux density, is shown as a green solid line. Therefore, the area of the hatched region corresponds to the integral part in the equation above. As can be seen, the ambipolar conductivity is restricted by minor carriers. To enhance the oxygen flux density, the minor conductivity should be enhanced. In addition to that, it is useful to reduce the membrane thickness to increase the oxygen flux density, unless the surface exchange reaction limits the oxygen evolution rate.
Another important issue is the chemical stability. When the oxygen permeable ceramics is utilized for the natural gas reforming, the material is exposed to a reducing atmosphere at the syngas side and oxidizing atmosphere at the opposite side, simultaneously. That is to say, large oxygen partial pressure gradient of about 0.21~10-20 atm exists between surfaces. Therefore, the chemical stability under such severe conditions is required, in addition to the high oxygen flux density. We are looking for novel materials with the high oxygen permeation properties and chemical stability. For examples, alkaline earth – indium - transition metal systems with perovskite-type structure and composites of doped ceria and electronic conductive oxides are under investigation. Moreover, thin film preparation using a pulsed-laser deposition technique is also being conducted.
References
- Y. Teraoka, H. M. Zhang, S. Furukawa, and N. Yamazoe: Oxygen permeation thorough perovskite-type oxides, Chem. Lett., (1985) 1743 – 1746.
- U. Balachandran, J. T. Dusek, S.M. Sweeney, R.B. Poeppel, R.L. Mieville, P.S. Maiya, M.S. Kleefisch, S. Pei, T.P. Kobylinski, C.A. Udovich, and A.C. Bose: Methane to syngas via Ceramic Membranes, Am. Ceram. Soc. Bull., 74 (1995) 71 – 75.
- U. Balachandran, J.T. Dusek, P.S. Maiya, B. Ma, R.L. Mieville, M. S. Kleefisch, and C. A. Udovich: Ceramic membrane reactor for converting methane to syngas, Catal. Today, 36 (1997) 265 – 272.
- R. Bredesen and T. Norby: On phase relations, transport properties and defect structure in mixed conducting SrFe1.5-xCoxOz, Solid State Ionics, 129 (2000) 285 – 297.
- T. Ishihara, T. Yamada, H. Arikawa, H. Nishiguchi, Y. Takita: Mixed electronic-oxide ionic conductivity and oxygen permeating property of Fe-, Co-, or Ni-doped LaGaO3 perovskite oxide, Solid State Ionics, 135 (2000) 631 – 636.
- H. J. M. Bouwmeester and A. J.Burggraaf, “Dense Ceramic Membranes for Oxygen Separation,” in The CRC Handbook of Solid State Electrochemistry, ed. by P. J. Gellings and H. J. M. Bouwmeester, CRC Press, New York, 1996, p. 481-553.
|
Copyright(c) 2002-2005 Hitoshi Takamura. All rights Reserved.
|
|